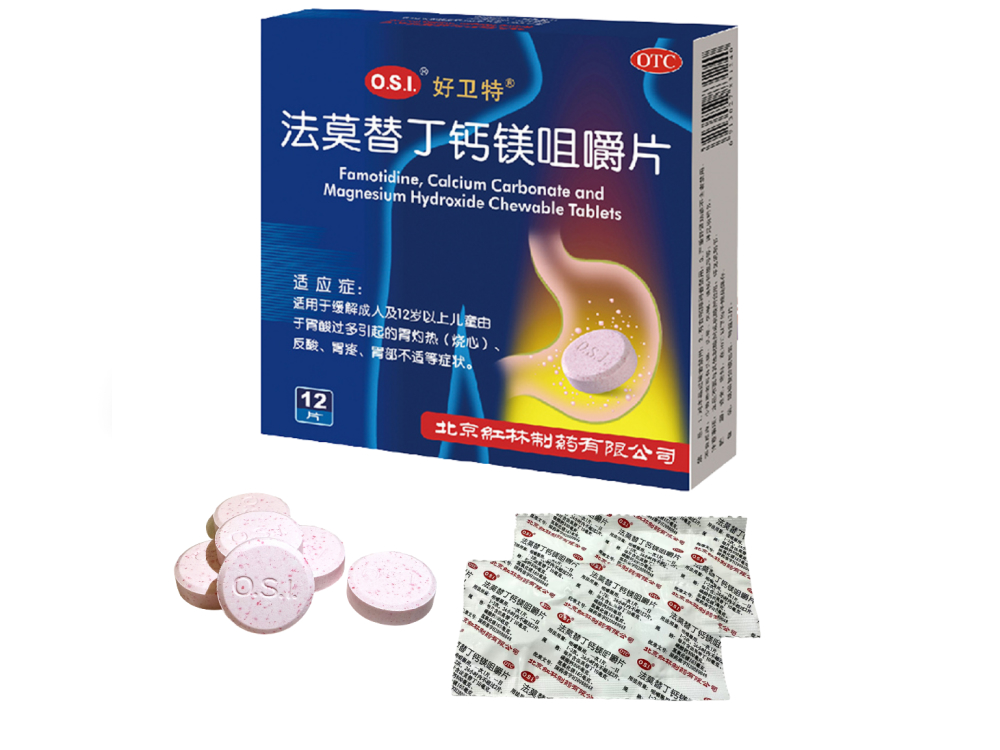
Howelt® Famotidine, Calcium Carbonate and Magnesium Hydroxide Chewable Tablets is the core product of our company in the digestive field. Initially available as a prescription drug, it could only be dispensed in hospitals. As its advantages of “safety, targeted efficacy, and convenient administration” became increasingly prominent in clinical application, our company took the initiative to apply for the prescription-to-OTC switch in 2014. After a series of processes, the application was successfully approved. Howelt® has transformed from an “exclusive medication” only available in hospitals to a common medication accessible to the public in pharmacies.
What’s more noteworthy is that Howelt® has passed the rigorous screening of Johnson & Johnson, a global pharmaceutical giant (including its subsidiary Xian-Janssen), by virtue of its excellent quality, officially becoming a strategic cooperation product between our company and Johnson & Johnson. Howelt®’s success in standing out among numerous similar drugs lies in three key advantages: first, a scientific formula that quickly relieves symptoms such as heartburn and acid reflux caused by excess stomach acid; second, strict production control ensuring extremely stable quality of each batch; third, reliable clinical data, with few adverse reactions verified through years of clinical application, making it a safe choice for patients.
Through Johnson & Johnson’s mature channels and brand influence, Howelt® can benefit more patients. This cooperation not only reflects Johnson & Johnson’s recognition of Howelt®’s value but also demonstrates our company’s R&D strength in the field of compound preparations and capabilities in international cooperation.
2021 官网升级中!现在您访问官网的浏览器设备分辨率宽度低于1280px请使用高分辨率宽度访问。