GoeGoe Honglin’s main products include: antihypertensive drug JUPOPINE® (Nifedipine Controlled-Release Tablets), antidiabetic drug RoToFier® (Glipizide Extended Release Tablets), antihypertensive drug JUPOSHU® (Amlodipine Besylate Tablets), and analgesic drug JUPOPHON® (Ibuprofen Sustained-Release Capsules). All the four aforementioned products have passed the Consistency Evaluation of Generic Drugs in Quality and Efficacy, among which JUPOPINE® and RoToFier® were among the first in China to obtain this certification.
All the above products have been included in national and alliance-based centralized drug procurement and are supplied in sufficient quantities. JUPOPINE® was selected in the 7th round of national centralized drug procurement. RoToFier®, JUPOPHON®, and JUPOSHU® participated in the successor projects of national centralized drug procurement: RoToFier® won the bid for the national market, while JUPOPHON® and JUPOSHU® were selected in 26 provinces and municipalities (all the above-mentioned selected regions include Jiangsu Province).
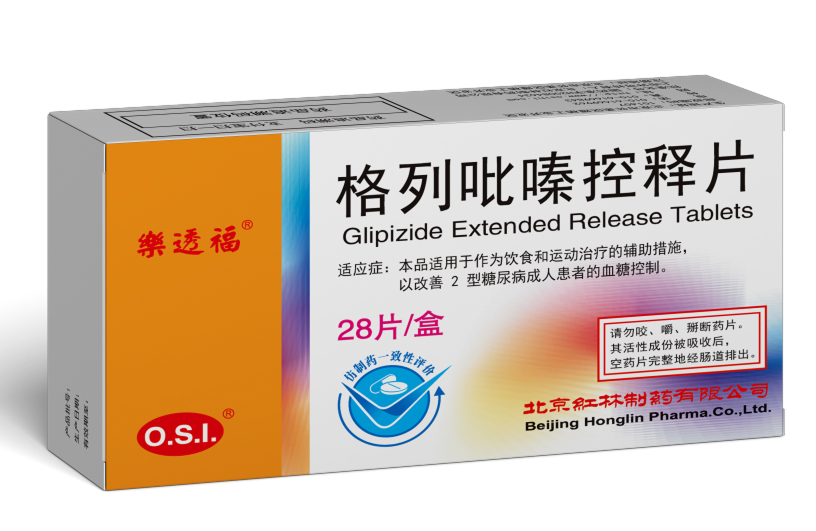
Product Recognition
On March 12, 2021, the antidiabetic drug RoToFier® (Glipizide Extended Release Tablets) was among the first in China to
pass the Generic Drug Consistency Evaluation.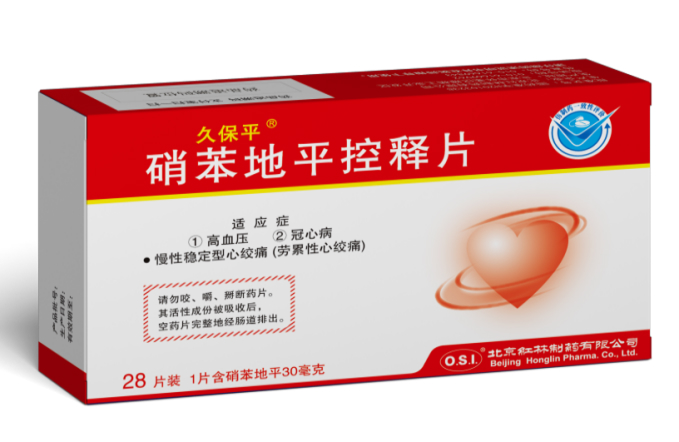 On April 30, 2021, the antihypertensive drug JUPOPINE® (Nifedipine Controlled-Release Tablets) was among the first in China to pass the Generic Drug Consistency Evaluation, and was officially designated as “Nifedipine Controlled-Release Tablets.”
On April 30, 2021, the antihypertensive drug JUPOPINE® (Nifedipine Controlled-Release Tablets) was among the first in China to pass the Generic Drug Consistency Evaluation, and was officially designated as “Nifedipine Controlled-Release Tablets.”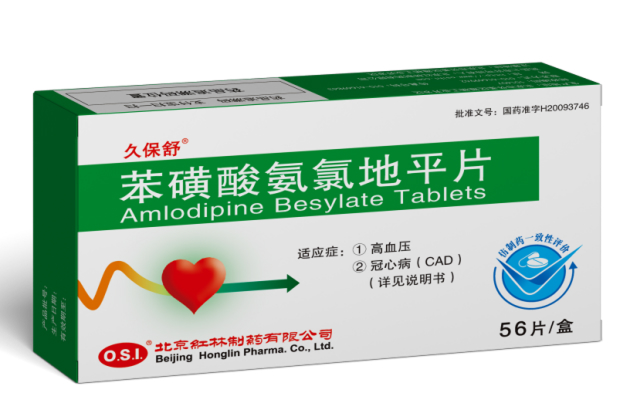 On May 24, 2021, the antipyretic-analgesic drug JUPOSHU® (Amlodipine Besylate Tablets) officially passed the Generic Drug Consistency Evaluation.
On May 24, 2021, the antipyretic-analgesic drug JUPOSHU® (Amlodipine Besylate Tablets) officially passed the Generic Drug Consistency Evaluation.
2021 官网升级中!现在您访问官网的浏览器设备分辨率宽度低于1280px请使用高分辨率宽度访问。